Nitrogen oxides

The term nitrogen oxides (NOx) describes a mixture of nitric oxide (NO) and nitrogen dioxide (NO2), which are gases produced from natural sources, motor vehicles and other fuel burning processes.
Nitric oxide is colourless and is oxidised in the atmosphere to form nitrogen dioxide. Nitrogen dioxide has an odour, and is an acidic and highly corrosive gas that can affect our health and environment.
Nitrogen oxides are critical components of photochemical smog. They produce the yellowish-brown colour of the smog.
In poorly ventilated situations, indoor domestic appliances such as gas stoves and gas or wood heaters can be significant sources of nitrogen oxides.
Environmental and health effects of nitrogen oxides
Elevated levels of nitrogen dioxide can cause damage to the human respiratory tract and increase a person's vulnerability to, and the severity of, respiratory infections and asthma.
Long-term exposure to high levels of nitrogen dioxide can cause chronic lung disease.
It may also affect the senses, for example, by reducing a person's ability to smell an odour.
High levels of nitrogen dioxide are also harmful to vegetation—damaging foliage, decreasing growth or reducing crop yields.
Nitrogen dioxide can fade and discolour furnishings and fabrics, reduce visibility, and react with surfaces.
Air quality guidelines
From 30 August 2024, the Environmental Protection (Air) Amendment Policy 2024 (EPP Air) objectives for nitrogen dioxide are:
- 0.08 parts per million (ppm) for a 1-hour exposure period
- 0.015ppm for an annual exposure period.
The National Environment Protection (Ambient Air Quality) Measure standards for nitrogen dioxide are:
- 0.08ppm for a 1-hour exposure period
- 0.015ppm for an annual exposure period.
These guidelines are designed to protect sensitive individuals, such as children and asthmatics.
Typical outdoor nitrogen dioxide levels are well below the 1-hour guidelines and exposure at these levels does not generally increase respiratory symptoms.
Measuring nitrogen oxides
Nitrogen oxides are measured with a technique known as ‘chemiluminescence’, which is a chemical reaction that emits energy in the form of light.
This particular reaction is the oxidation of nitric oxide (NO) to nitrogen dioxide (NO2) by ozone (O3) as shown below:
NO + O3 > NO2*+ O2
It is an exothermic (heat generating) reaction, which produces an activated molecule of NO2*. When these NO2* molecules return from the activated state to normal state, some energy is emitted in the form of a small amount of light. A photomultiplier tube measures the intensity of the emitted light.
Since 1 NO molecule is required to form 1 NO2 molecule, the intensity of the chemiluminescent reaction is directly proportional to the NO concentration in the sample. The analyser measures the amount of light emitted and converts this to a concentration.
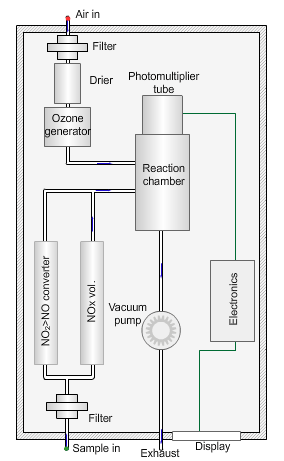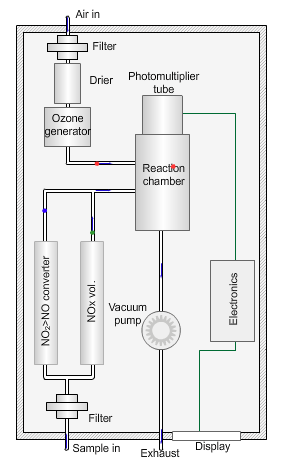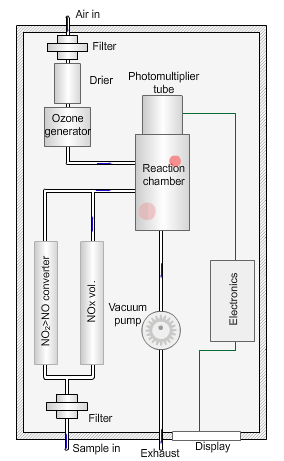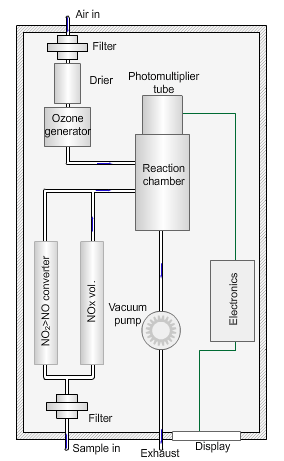

Nitrogen oxides analyser
The animated illustration shows how the analyser works.
A vacuum pump draws both the air supply for the ozone generator and the ambient air samples into the analyser. The green dot shows the ambient air sample path. A high-voltage corona discharge generates the ozone in dry air, shown in the diagram by the path of the red dot.
The chemiluminescent reaction only occurs between O3 and NO.
To measure the NO2 component the instrument diverts the ambient air stream alternately through a converter containing a catalyst (molybdenum) maintained at a temperature of 315°C, which converts any NO2 present to NO before entering the reaction cell.
The blue dot shows the path taken. The difference between NO levels in the undiverted and diverted gas streams is the amount of NO2.
Other instruments
Differential optical absorption spectroscopy (DOAS) instruments also measure nitrogen oxides.
More information
Go to the live air data service to check the current levels of nitrogen oxides in the monitoring network.


