Sulfur dioxide

Sulfur dioxide molecule
Sulfur dioxide (SO2) is a colourless gas with a sharp, irritating odour. It is produced by burning fossil fuels and by the smelting of mineral ores that contain sulfur.
Erupting volcanoes can be a significant natural source of sulfur dioxide emissions.
Environmental effects
When sulfur dioxide combines with water and air, it forms sulfuric acid, which is the main component of acid rain. Acid rain can:
- cause deforestation
- acidify waterways to the detriment of aquatic life
- corrode building materials and paints.
In Queensland, there is less heavy industry than in Europe or North America, where the potential for forming acid rain from sulfur dioxide emissions is higher. Our weather conditions and low sulfur content of fuels reduce the potential for acid rain.
Health effects
Sulfur dioxide affects the respiratory system, particularly lung function, and can irritate the eyes.
Sulfur dioxide irritates the respiratory tract and increases the risk of tract infections. It causes coughing, mucus secretion and aggravates conditions such as asthma and chronic bronchitis.
Air quality guidelines
From 1 January 2025, the Environmental Protection (Air) Amendment Policy 2024 (EPP Air) objectives for sulfur dioxide are:
- 0.075 parts per million (ppm) for a 1-hour exposure period
- 0.02ppm for a 24-hour exposure period
From 1 January 2025, the National Environment Protection (Ambient Air Quality) Measure standards for sulfur dioxide are:
- 0.075 parts per million (ppm) for a 1-hour exposure period
- 0.02ppm for a 24-hour exposure period
These guidelines are designed to protect sensitive individuals, such as children and asthmatics.
Significant concentrations of sulfur dioxide are only measured in Queensland near large industrial sources.
Measuring sulfur dioxide
Sulfur dioxide analyser
The animated illustration shows how the sulfur dioxide analyser works.
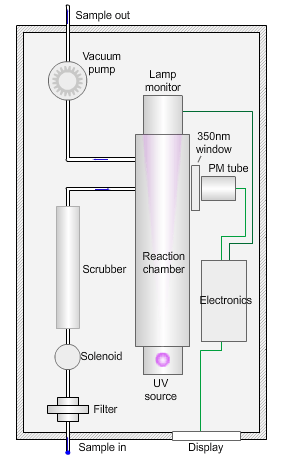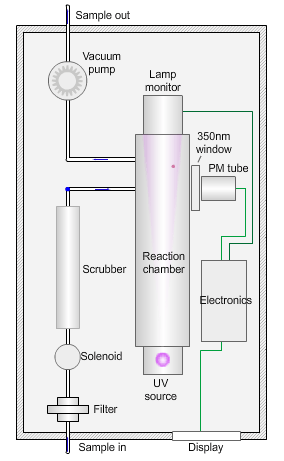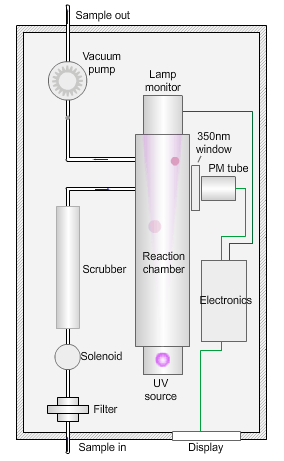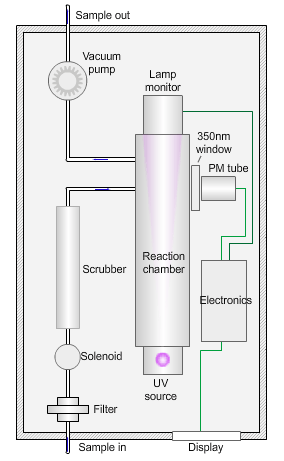
A sulfur dioxide analyser
The sample (shown by the path of the blue dot) is drawn into the analyser by means of the vacuum pump, firstly through a filter to remove particles, and then through a scrubber to remove interfering gases, such as hydrocarbons.
The scrubbed sample passes into a reaction chamber where it is irradiated with ultraviolet (UV) light at 214nm (nanometres) generated by a zinc discharge lamp and a UV bandpass filter.
Sulfur dioxide absorbs UV radiation at wavelengths between 200nm and 240nm. Emission of fluorescence (light-producing) photons at higher wavelengths (around 350nm) follows this absorption of UV radiation by the molecule.
This fluorescence is measured perpendicular to the beam using a photomultiplier (PM) tube and the signal converted to a concentration value.
The measured fluorescence is directly proportional to the concentration of sulfur dioxide in the sample.
Other instruments
Differential Optical Absorbance Spectroscopy (DOAS) instruments also measure sulfur dioxide.
More information
Go to the live air data service to check current levels of sulfur dioxide in the monitoring network.