Ozone
Ozone is a colourless, highly reactive gas with a distinctive odour.
It is formed naturally by electrical discharges (for example, lightning) and in the upper atmosphere at altitudes of 15–35km.
The upper atmosphere ozone layer protects the earth from harmful ultraviolet radiation from the sun. The ozone layer reduction represents a global atmosphere issue.
Photochemical smog
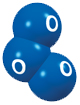
At ground level, elevated concentrations of ozone are produced by chemical reactions between sunlight and certain air pollutants.
Given sunlight and suitable weather conditions, nitrogen oxides and volatile organic compounds (VOCs) can react to form photochemical oxidants, commonly referred to as photochemical smog, of which ozone is the major component.
The reactions that produce photochemical oxidants usually occur during strong sunlight over several hours. Highest concentrations are normally measured on summer afternoons in areas downwind from the sources of ozone precursors (nitrogen oxides and VOCs).
Combustion processes (including motor vehicle engines, power stations or bushfires) are the major sources of nitrogen oxides and VOCs.
Bushfires generate large quantities of the pollutants that form ozone.
In recent years, ozone levels higher than guideline values in South East Queensland have usually been associated with bushfires or burning-off events during calm weather conditions. Rather than being dispersed by winds the stable weather conditions allow the bushfire emissions to build up.
Environmental and health effects of ozone
At ground level, elevated ozone concentrations can cause health and environmental problems.
Ozone can affect the cardiac system and irritate the respiratory tract when present at concentrations significantly above natural levels. Symptoms of exposure include:
- itching and watery eyes
- sore throats
- swelling and congestion of the nasal passages.
At elevated levels, ozone can also:
- reduce vegetation growth
- damage materials such as rubber, fabric, masonry and paint
- reduce visibility.
Air quality guidelines
From 30 August 2024, the Environmental Protection (Air) Amendment Policy 2024 (EPP Air) objective for ozone is:
- 0.065 parts per million (ppm) for an 8-hour exposure period
The National Environment Protection (Ambient Air Quality) Measure standard for ozone is:
- 0.065ppm for an 8-hour exposure period
These guidelines are designed to keep ozone concentrations below the levels that affect sensitive members of the population.
Measuring ozone
Instruments that monitor ground-level ozone concentrations use the principle of absorption of ultraviolet (UV) light.
Ozone exhibits strong absorption in UV spectrum at around 250nm (nanometres). This is how the ozone layer protects the earth from the UV radiation emitted from the sun.
Ozone analyser
The animated illustration shows how an ozone analyser works.
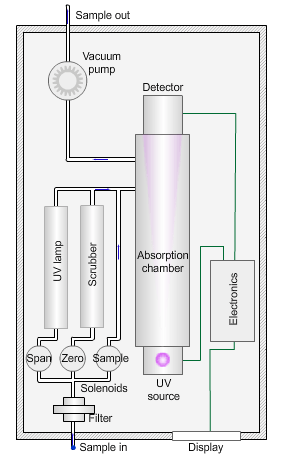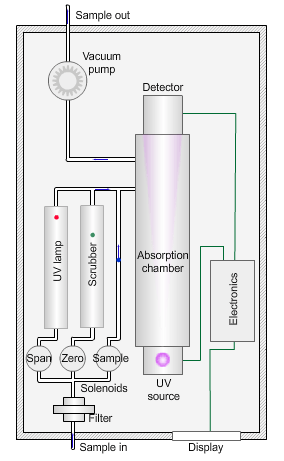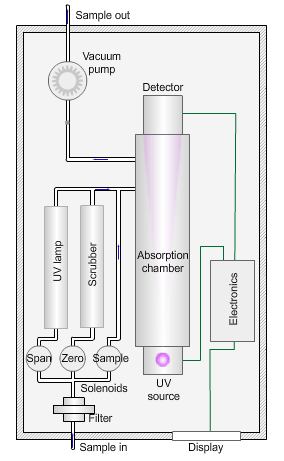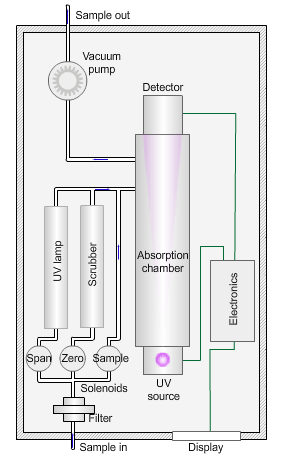
To determine the ozone concentration, a pump draws a sample of ambient air through a filter and into an absorption chamber where UV light of wavelength 254nm from a mercury vapour lamp irradiates the sample.
The blue dot in the diagram shows the sample path. The instrument detects a reduction in the intensity of the UV light relative to the concentration of ozone in the sample.
The instrument then measures a ‘zero’ sample with all ozone removed by a manganese dioxide scrubber (shown by the path of the green dot).
The zero concentration results in a higher light intensity reaching the detector. The difference between the two values yields the concentration of ozone in the sample.
Finally, the instrument measures a sample irradiated by UV light of wavelength 185nm to generate ozone (shown by the path of the red dot). Comparison with a laboratory standard ensures the instrument is responding correctly.
The sampling pump then removes the exhaust gases (the grey dot) from the absorption chamber.
Other instruments
Differential Optical Absorption Spectroscopy (DOAS) instruments also measure ozone.
More information
Go to the live air data service to check the current levels of ozone in the monitoring network.